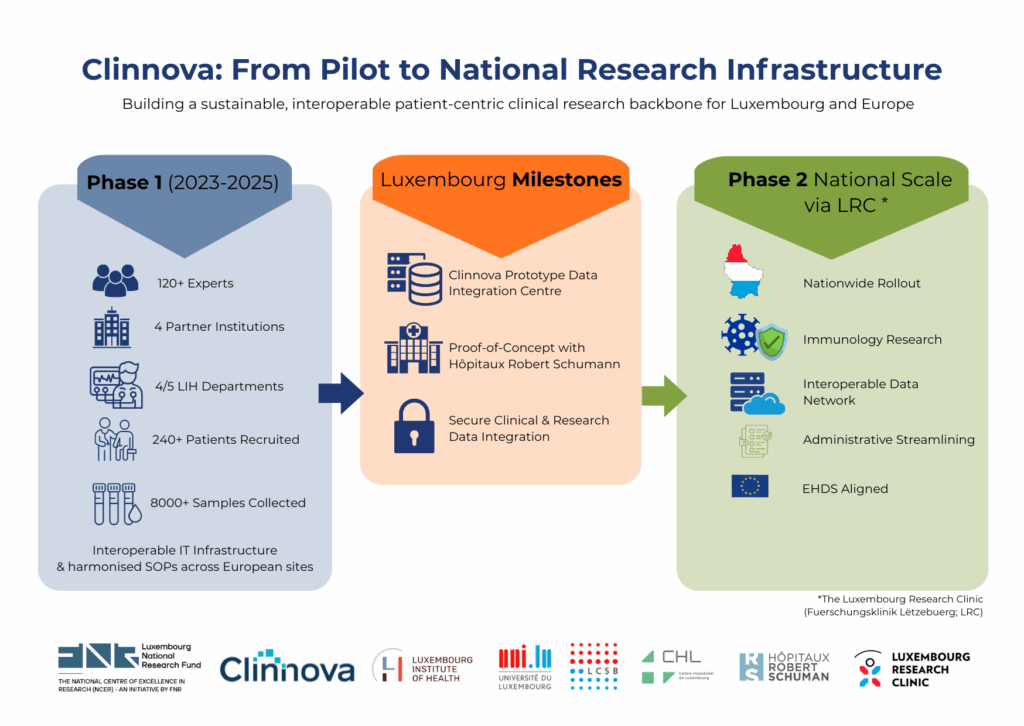
Research
Dedicated to life
At the Luxembourg Institute of Health (LIH), we believe we have a collective obligation towards society to use knowledge and technology arising from outstanding research to have a direct and meaningful impact on people’s health.
WE PUT THE PATIENT AT THE HEART OF EVERYTHING WE DO.
We perform patient-centric translational research with a focus on cancer and immune-related disorders. Our particular interest lies in the immune system as a shared functional module between health and disease.
Our dedicated teams of multidisciplinary researchers embrace collaboration and disruptive technology to advance the understanding of disease mechanisms. Using technology like Artificial Intelligence on real-world patient-derived data, we create disease relevant knowledge, which can be tangibly translated into clinical applications through a bed to bench to bed approach.
As a public biomedical research organisation focused on precision health, the LIH is invested in becoming a leading reference in Europe for the transformation of scientific excellence into meaningful benefits for patients.


How we are helping
Our patient-centric research puts people’s health at its heart.
Discover our priority disease areas :

How we are driving innovation
LIH is invested in becoming a reference in Europe for the translation of scientific excellence and innovation into meaningful benefits for patients.
LIH is invested in becoming a reference in Europe for the translation of scientific excellence and innovation into meaningful benefits for patients.
Discover our priority topics :

Contribute
As a patient, researcher, private company or donor, you too can get involved in our work and contribute to our mission in different ways
latest news
Forthcoming events
-

Lecture series – Epidemiology & Prevention 2025-2026
Epidemiology & Prevention
05/03/2026 10:00
-

🇬🇧 A family affair? Long-term economic and mental health effects of spousal cancer
Epidemiology & Prevention
Webinar
Speaker: Professor Petri Böckerman
05/03/2026 11:00
-

🇬🇧 Structural basis of glycan diversity in biological systems
Omics meets physics
Speaker: Prof Marcelo Guerin
10/03/2026 10:30
-

🇬🇧 Epigenomic evolution of breast cancers at single cell resolution
Lecture Series Cancer Research
Webinar
Speaker: Prof. Celine Vallot
12/03/2026 11:00
-

🇬🇧 Mechanisms of host-microbiota interactions in extraintestinal autoimmunity
Pathogenesis in the age of the microbiome
Speaker: Prof. Dr. Martin Kriegel
26/03/2026 10:30
-

🇬🇧 EIMN 2026 – 2nd European Immunometabolism conference -Decoding the Interplay Between Metabolism and Immunity
Speaker: Various
10/06/2026 11:00


Welcome to LIH
We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. By clicking “Accept All”, you consent to the use of ALL the cookies. However, you may visit "Cookie Settings" to provide a controlled consent. For more information about how LIH processes your personal data please click here.
Read More Cookie Settings Accept AllReject All
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| _GRECAPTCHA | 5 months 27 days | This cookie is set by the Google recaptcha service to identify bots to protect the website against malicious spam attacks. |
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Analytics" category . |
| cookielawinfo-checkbox-functional | 1 year | The cookie is set by the GDPR Cookie Consent plugin to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Necessary" category . |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to store the user consent for cookies in the category "Others". |
| cookielawinfo-checkbox-performance | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to store the user consent for cookies in the category "Performance". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| JSESSIONID | session | The JSESSIONID cookie is used by New Relic to store a session identifier so that New Relic can monitor session counts for an application. |
| PHPSESSID | session | This cookie is native to PHP applications. The cookie is used to store and identify a users' unique session ID for the purpose of managing user session on the website. The cookie is a session cookies and is deleted when all the browser windows are closed. |
| viewed_cookie_policy | 1 year | The cookie is set by the GDPR Cookie Consent plugin to store whether or not the user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| bcookie | 1 year | LinkedIn sets this cookie from LinkedIn share buttons and ad tags to recognize browser ID. |
| bscookie | 1 year | LinkedIn sets this cookie to store performed actions on the website. |
| lang | session | LinkedIn sets this cookie to remember a user's language setting. |
| li_gc | 5 months 27 days | Linkedin set this cookie for storing visitor's consent regarding using cookies for non-essential purposes. |
| lidc | 1 day | LinkedIn sets the lidc cookie to facilitate data center selection. |
| pll_language | 1 year | The pll _language cookie is used by Polylang to remember the language selected by the user when returning to the website, and also to get the language information when not available in another way. |
| UserMatchHistory | 1 month | LinkedIn sets this cookie for LinkedIn Ads ID syncing. |
| vOTSXIlGdxFUt | 1 day | No description |
| xwNvEASRYf_ | 1 day | No description |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 2 years | The _ga cookie, installed by Google Analytics, calculates visitor, session and campaign data and also keeps track of site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognize unique visitors. |
| _ga_ZM94YP9CD3 | 2 years | This cookie is installed by Google Analytics. |
| _gat_gtag_UA_16961320_1 | 1 minute | Set by Google to distinguish users. |
| _gid | 1 day | Installed by Google Analytics, _gid cookie stores information on how visitors use a website, while also creating an analytics report of the website's performance. Some of the data that are collected include the number of visitors, their source, and the pages they visit anonymously. |
| AnalyticsSyncHistory | 1 month | Linkedin set this cookie to store information about the time a sync took place with the lms_analytics cookie. |
| CONSENT | 2 years | YouTube sets this cookie via embedded youtube-videos and registers anonymous statistical data. |
| Cookie | Duration | Description |
|---|---|---|
| _fbp | 3 months | This cookie is set by Facebook to display advertisements when either on Facebook or on a digital platform powered by Facebook advertising, after visiting the website. |
| fr | 3 months | Facebook sets this cookie to show relevant advertisements to users by tracking user behaviour across the web, on sites that have Facebook pixel or Facebook social plugin. |
| IDE | 1 year 24 days | Google DoubleClick IDE cookies are used to store information about how the user uses the website to present them with relevant ads and according to the user profile. |
| test_cookie | 15 minutes | The test_cookie is set by doubleclick.net and is used to determine if the user's browser supports cookies. |
| VISITOR_INFO1_LIVE | 5 months 27 days | A cookie set by YouTube to measure bandwidth that determines whether the user gets the new or old player interface. |
| YSC | session | YSC cookie is set by Youtube and is used to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
| yt-remote-device-id | never | YouTube sets this cookie to store the video preferences of the user using embedded YouTube video. |
| yt.innertube::nextId | never | This cookie, set by YouTube, registers a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | This cookie, set by YouTube, registers a unique ID to store data on what videos from YouTube the user has seen. |
| Cookie | Duration | Description |
|---|---|---|
| DEVICE_INFO | 5 months 27 days | No description |
| ln_or | 1 day | No description |