Clinical and epidemiological investigation centre
The CIEC supports research and health care institutions, international academia and pharmaceutical industry players, providing them with high quality clinical research and services.
Missions
The Clinical and Epidemiological Investigation Center (CIEC), as part of the Translational Medicine Operations Hub (TMOH), is establishing a growing reputation in clinical research. Acting as a national centre coordinating clinical research activities involving clinicians in various medical fields, CIEC is a contact partner for pharmaceutical industries interested in conducting clinical trials in Luxembourg and works very closely with the Clinical Projects Management Office (CPMO) and the Competence Centre for Methodology and Statistics (CCMS). CIEC stands for excellence in operational support in clinical research whilst striving to ensure respect of patient rights, data privacy and offering the opportunity to access new, innovative therapeutic approaches otherwise inaccessible.
CIEC supports research and health care institutions, international academia and pharmaceutical industry players, providing them with high quality clinical research and services. CIEC coordinated more than 7000 participants included in 140 research projects, academic or pharma-led clinical trials in the last 10 years and further aims at better addressing patients’ needs and quality of life by increasing relevant and innovative clinical research in Luxembourg, including the development of clinical and translational research.
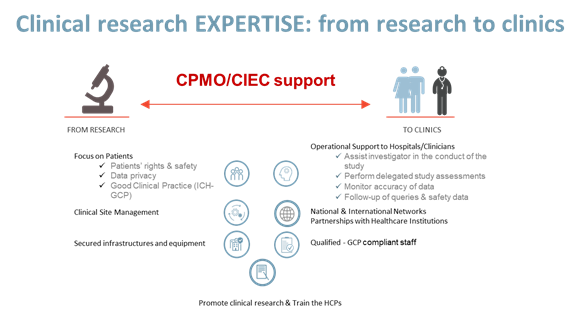
| PROVIDE | PROTECT | PROMOTE | PARTICIPATE |
| Operational & educational support to hospitals and health care professionals | Patients’ rights & safety | Communication and valorization of Clinical Research | Good Clinical Practice (ICH-GCP) |
| Access to new and innovative therapeutic approaches/strategies for patients | Data privacy | Clinical Research according to Good Clinical Practice (ICH-GCP) | Networks: ECRIN, EFGCP, EUPATI…. |
| Clinical Research Network | Good Clinical Practice (ICH-GCP) | Research Integrity & Ethics | Education initiatives (Chercheurs à l’école) |
Partners
Projects & clinical trials

Featured team members
Scientific publications
-
Penetrance of Parkinson’s disease in GBA1 carriers depends on variant severity and polygenic background – 12/06/2025
-
Interpretable Machine Learning for Cross-Cohort Prediction of Motor Fluctuations in Parkinson’s Disease – 22/04/2025
-
Dopamine Pathway and Parkinson’s Risk Variants Are Associated with Levodopa-Induced Dyskinesia – 01/01/2024
-
Integrating digital gait data with metabolomics and clinical data to predict outcomes in Parkinson’s disease – 06/09/2024
-
Levodopa-induced dyskinesia in Parkinson’s disease – 01/09/2024
-
Mixed effects models but not t-tests or linear regression detect progression of apathy in Parkinson’s disease over seven years in a cohort – 24/08/2024
-
Cardiovascular history and risk of idiopathic Parkinson’s disease – 08/07/2024
-
Converging peripheral blood microRNA profiles in Parkinson’s disease and progressive supranuclear palsy – 31/05/2024
-
Gut microbiome is not associated with mild cognitive impairment in Parkinson’s disease – 06/04/2024
-
Comprehensive blood metabolomics profiling of Parkinson’s disease reveals coordinated alterations in xanthine metabolism – 19/03/2024
Related News

Job vacancies
-
Clinical Research Nurse (JA/CRN0625/JG/CRNT)
Translational Medicine Operations Hub – Clinical & Epidemiological Investigation Center