BIOSPECIMEN PROFICIENCY TESTING
We provide a Proficiency Testing (PT) programme to laboratories working with biospecimens, including biorepositories, clinical laboratories, research organisations and bioservice providers. Our research focus on biospecimen science, our dedication to quality and our range of in-house services make us an ideal partner for PT. Our PT programme is endorsed by the International Society for Biological and Environmental Repositories (ISBER).
Validate your routine lab methods
The PT programme provides an external quality assessment tool to verify the precision and accuracy of your biospecimen testing methods, and the efficiency of your processing methods.
Compare your performance to others
The principle of performance benchmarking applied in our PT programme means that your results will be compared to those of other laboratories, from different sectors all over the world.
Comply with certification & accreditation requirements
Proficiency testing offers organisations that are seeking compliance with ISO 20387, ISO 17025, ISO 15189, CLIA, CAP or equivalent, the opportunity to fulfill the respective normative requirements.
Gain more credibility & visibility
By participating in our PT programme you can assure customers, collaborators, funding agencies and regulatory bodies of the validity of your results. This can help build your reputation for high quality laboratory methods and gain international visibility.
Improve performance & prove consistency
The assessment of your laboratory’s proficiency allows you to monitor and improve performance by identifying potential problems and providing additional motivation. Regular participation in our PT programme will also prove consistency of performance over time.
Our 2025 Proficiency Testing Schemes
The 2025 PT programme includes 22 processing and testing schemes.
COMBINED PROCESSING AND TESTING SCHEMES:
CIRCULATING TUMOR CELLS (CTC) ISOLATION AND DETECTION
- VyCAP system
- Siemens system with Whatman membranes
PROCESSING SCHEMES
DUAL DNA/RNA EXTRACTION FROM FROZEN TISSUE
- Magnetic bead-based
- Silica membrane-based
- Salting out
- Phenol Trizol
CSF ALIQUOTING
- Aliquoting
VIABLE PBMC ISOLATION
- Manual extraction
- Automated extraction
DNA EXTRACTION FROM BUFFY COAT
- Magnetic bead-based
- Silica membrane-based
- Salting out
DNA EXTRACTION FROM WHOLE BLOOD
- Magnetic bead-based
- Silica membrane-based
- Salting out
DNA EXTRACTION FROM FFPE MATERIAL
- Magnetic bead-based
- Silica membrane-based
- Salting out
DNA EXTRACTION FROM FROZEN TISSUE
- Magnetic bead-based
- Silica membrane-based
- Salting out
MICROBIAL DNA EXTRACTION FROM SALIVA
- Magnetic bead-based
- Silica membrane-based
- Salting out
MICROBIAL DNA EXTRACTION FROM STOOL
- Magnetic bead-based
- Silica membrane-based
- Salting out
CELL FREE DNA (cfDNA) EXTRACTION FROM WHOLE BLOOD
- Magnetic bead-based
- Silica membrane-based
- Salting out
RNA EXTRACTION FROM BUFFY COAT
- Magnetic bead-based
- Silica membrane-based
- Phenol Trizol
RNA EXTRACTION FROM WHOLE BLOOD
- Magnetic bead-based
- Silica membrane-based
- Phenol Trizol
RNA EXTRACTION FROM FFPE MATERIAL
- Magnetic bead-based
- Silica membrane-based
- Phenol Trizol
TOTAL RNA EXTRACTION FROM FROZEN TISSUE
- Magnetic bead-based
- Silica membrane-based
- Phenol Trizol
CELL FREE RNA (cfRNA) EXTRACTION FROM PLASMA
- Magnetic Bead-Based
- Silica Membrane-Based
- Trizol
TESTING SCHEMES
DNA QUANTIFICATION AND PURITY
- Spectrophotometry (ng/µl and 260/280 ratio)
- Spectrofluorometry (ng/µl)
- Microfluidics LabOnchip (ng/µl)
- Lunatic Spectrophotometry (ng/µl and 260/280 ratio)
RNA QUANTIFICATION AND PURITY
- Spectrophotometry (ng/µl and 260/280 ratio)
- Spectrofluorometry (ng/µl)
- Microfluidics LabOnchip (ng/µl)
- Lunatic Spectrophotometry (ng/µl and 260/280 ratio)
RNA INTEGRITY
- Agilent Bioanalyzer (RIN)
- Biorad Experion (RQI)
- Agilent TapeStation Systems (SDV)
- Fragment Analyzer (RQN)
- QIAxcel System (RIS)
- Caliper LabChip GX (RQS)
- Qubit 4 Fluorometer (RNA IQ)
CELL VIABILITY
- Staining-based (e.g. trypan blue)
- Flow Cytometry
DNA INTEGRITY
- Agilent TapeStation (DIN)
- Caliper LabChip GX (GQS)
- Fragment Analyzer (GQN)
- QIAxcel System
TISSUE HISTOLOGY
- Tissue characterization/mapping and assessment of tissue tumor content
Key dates to keep in mind
12 May 2025: Registration opens online
31 August 2025: Registration closes
October 2025: Standardized samples will be shipped to your location
November 2025: Data submission deadline
March 2026: Your report(s) will be sent electronically
Pricing
The prices indicated in the table below are per individual scheme.
ISBER members benefit from a 15% discount on all the listed fees. You can participate in one or more PT schemes.
The consecutive years registration is discontinued. Previously registered participants retain the right to use their remaining rounds within a maximum period of three consecutive years.
More details will be shared to the participants directly.
The table below summaries the fees (in EUR) for the 2025 PT programme:
| Schemes | Code | 2025 |
| DNA Quantification and Purity* | DNAQ25 | €579 |
| RNA Integrity* | RNAI25 | €579 |
| RNA Quantification and Purity* | RNAQ25 | €579 |
| Cell Viability* | CELL25 | €579 |
| DNA integrity* | DNAI25 | €579 |
| Tissue Histology | THIS25 | €469 |
| CSF Aliquoting | CSAL25 | €469 |
| DNA Extraction from Buffy Coat | DNABFF25 | €359 |
| DNA Extraction from Whole Blood | DNABLD25 | €359 |
| DNA Extraction from FFPE Material | DNAFFC25 | €359 |
| RNA Extraction from Buffy Coat | DNABFF25 | €359 |
| RNA Extraction from Whole Blood | RNABLD25 | €359 |
| RNA Extraction from FFPE Material | RNAFFC25 | €359 |
| Microbial DNA Extraction from Saliva | DNASAL25 | €359 |
| Microbial DNA Extraction from Stool | DNASTL25 | €359 |
| Cell Free DNA (cfDNA) Extraction from Whole Blood | cfDNA25 | €359 |
| DNA Extraction from Frozen Tissue | DNAFRT25 | €359 |
| Total RNA Extraction from Frozen Tissue | RNAFRT25 | €359 |
| Viable PBMC Isolation | PBMC25 | €469 |
| Cell Free RNA (cfRNA) Extraction from Plasma | cfRNA25 | €359 |
| Dual DNA/RNA Extraction from Frozen Tissue | DUALFRT25 | €359 |
| Circulating Tumor Cells (CTC) Isolation and Detection | CTC25 | €359 |
For the schemes with *, you may test multiple methods in the same run according to the lists of accepted methods. For all testing schemes, namely DNAQ25, RNAQ25, RNAI25, DNAI25, CELL25 and THIS25, you may enter up to 10 replicate measurements.
After registration closure, schemes with low number of registrations will not be conducted for statistical reasons.
No refunds are available after the registration deadline. Partial refunds are available for cancellation requests received in writing prior to the end of the registration period, after an administrative cancellation fee of 100€ (EUR) is applied.
French clients are normally eligible for tax relief of up to 50% on their R&D expenditure at IBBL. Read more about the Crédit d’impôt recherche.
Shipments of PT schemes form/to IBBL:
For the shipment of PT derivatives to IBBL, the cost will be covered by the participants.
For participants outside EU and US/Canada: the shipment effective cost of PT schemes from IBBL to participants will be covered by the participants. After registration, more details will be shared to the participants directly.
The street address on the registration form will be used as the SHIPPING address. If the samples should be shipped to a different address, please send that address to ISBERPT@lih.lu.


How does it work?
Our PT programme works as an external quality assessment tool that lets you verify and benchmark the performance of your biospecimen processing or testing methods through the following steps:
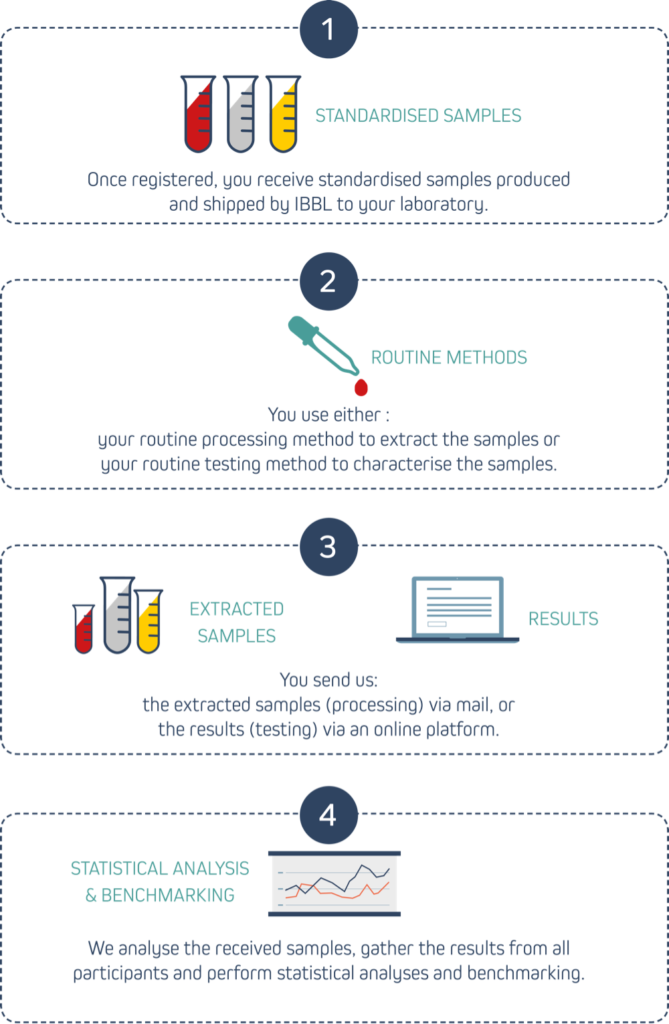
What do you receive?
In the end, each participant receives:
- a personalised report
- a certificate of participation
- a label of participation

Participants
If you have registered for the 2025 PT programme, you can access your account by clicking on the login button below. Please read the general Instructions for Participants.
You should also download the Participant Manual, the Instructions for Shipment (only for Processing Schemes) and the information sheets corresponding to the schemes you have signed up for:
- DNA Quantification and Purity – Test Item Information Sheet
- RNA Quantification and Purity – Test Item Information Sheet
- RNA Integrity – Test Item Information Sheet
- Cell Viability – Test Item Information Sheet
- DNA integrity – Test Item Information Sheet
- Tissue Histology – Test Item Information Sheet
- CSF Aliquoting – Processing Item Information Sheet & CSAL Instructions for Shipment
- Viable PBMC Isolation – Processing Item Information Sheet & PBMC Instructions for Shipment
- DNA Extraction from Whole Blood – Processing Item Information Sheet
- DNA Extraction from Buffy Coat – Processing Item Information Sheet
- DNA Extraction from FFPE Material – Processing Item Information Sheet
- DNA Extraction from Frozen Tissue – Processing Item Information Sheet
- Microbial DNA Extraction from Saliva – Processing Item Information Sheet
- Microbial DNA Extraction from Stool – Processing Item Information Sheet
- Cell Free DNA (cfDNA) Extraction from Whole Blood – Processing Item Information Sheet
- RNA Extraction from Whole Blood – Processing Item Information Sheet
- RNA Extraction from Buffy Coat – Processing Item Information Sheet
- RNA Extraction from FFPE Material – Processing Item Information Sheet
- Total RNA Extraction from Frozen Tissue – Processing Item Information Sheet
- Cell Free RNA (cfRNA) Extraction from Plasma – Processing Item Information Sheet
- Circulating Tumor Cells (CTC) Isolation and Detection – Processing Item Information Sheet
- Dual DNA/RNA Extraction from Frozen Tissue – Processing Item Information Sheet