News
Towards personalised treatment of recurrent brain tumours
- Department of Cancer Research
- Department of Infection and Immunity
- NORLUX Neuro-Oncology Laboratory
- Precision Health
- Cancer

The GLASS-LUX project receives funding from the FNR and the Fondation Cancer
On February 25th, Prof Simone Niclou, Director of the LIH Department of Oncology (DONC) and group leader of the NORLUX Neuro-Oncology Laboratory, was awarded a EUR 850,000 check by the Fondation Cancer and the Luxembourg National Research Fund (FNR) in support of the GLASS-LUX study, in the presence of Dr Marc Schiltz, CEO of the FNR, and Dr Bauer, president of the Fondation Cancer. GLASS-LUX (Glioma Longitudinal AnalySiS in Luxembourg: ex vivo and in vivo Functional Profiling of Recurrent Gliomas), which will be launched in the spring for a duration of 36 months, aims to characterise the molecular and genetic differences between primary and recurrent brain tumours, and test their differential responses to a broad selection of both novel and existing drugs. This will enable the prediction of personalised treatment options for recurrent glioma patients after the standard of care has been unsuccessful. The FNR/Fondation Cancer funding for GLASS-LUX was bestowed in parallel to two other financial commitments to two additional DONC projects under the 2020 FNR CORE programme.
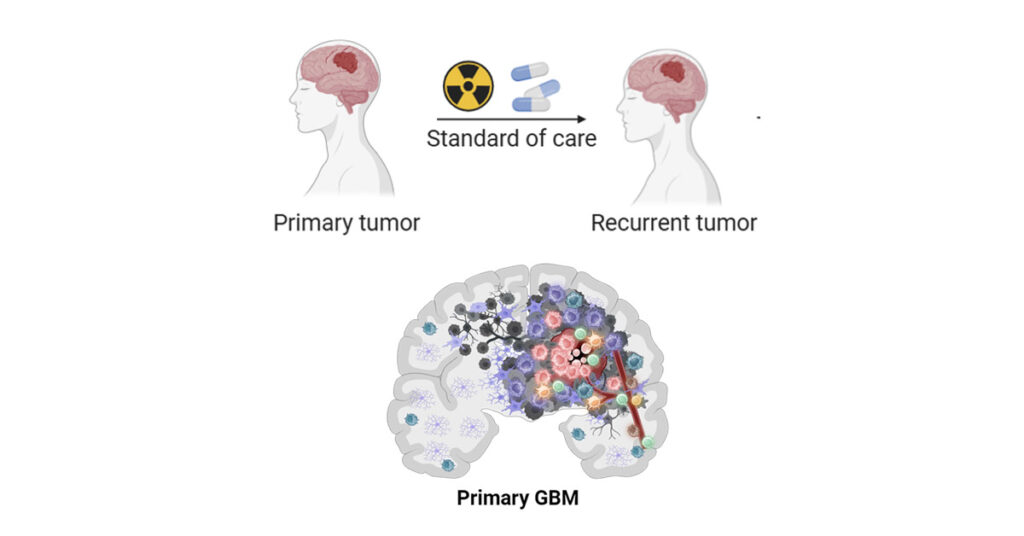
Adult diffuse gliomas are a particularly diverse group of primary brain tumours, with grade IV glioblastoma (GBM) being the most malignant subtype. Despite surgery, radiation and chemotherapy, the median survival of GBM patients is only about 14 months, with recurrence being inevitable. One of the main reasons for the poor prognosis of GBM patients is the high level of genetic alterations of this type of cancer, leading to the high proliferation and widespread invasion of tumour cells to healthy brain tissue, as well as strong resistance to treatment. Several studies suggest that diffuse gliomas evolve upon treatment and during recurrence, displaying molecular and functional differences compared to the original primary tumour. Additionally, recent evidence points towards the role of epigenetic modifications[1] such as DNA methylation in promoting resistance to treatment, making the tumour unresponsive to temozolomide (TMZ), the standard-of-care chemotherapeutic agent.
In this context, GLASS-LUX builds on the efforts of the international multi-institutional GLASS (Glioma Longitudinal AnalySiS) consortium, of which Prof Niclou is a member, which will provide a detailed molecular characterisation of paired primary and recurrent gliomas throughout multiple centres in the US and Europe. GLASS-LUX represents the Luxembourg branch of this initiative and aims to test the sensitivity of primary and recurrent gliomas to drugs and assess their differential response, in an approach known as personalised functional profiling (PFP).
Patient glioma tissue samples, both from the primary and recurrent tumours, will be collected and used to generate three-dimensional models called tumour organoids. These organoids will be subjected to PFP in an attempt to determine their drug response behaviour. At the same time, tumour organoids are implanted in the brains of immunodeficient mice to give rise to so-called Patient-Derived Orthotopic Xenografts (PDOXs), “avatars” of a patient’s tumour which faithfully reproduce its main biological, histological and genomic features. Currently, the scientists have already generated 14 PDOX models from glioma specimens obtained from 7 different patients, with samples received at two different time points from each patient, and plan to generate an additional 10 over the duration of the project. These will complement the existing live biobank already established together with the CHL, consisting of over 900 brain tumour samples of different types, grades and with different mutations. All newly collected samples and resulting PDOXs will be characterised from a molecular perspective to understand their genetic and epigenetic profiles, in collaboration with the Laboratoire national de santé (LNS) and the GLASS consortium.
Patient-derived 3D organoids and PDOX models of primary and recurrent tumours will then undergo an innovative high-throughput personalised functional profiling (PFP) approach, consisting in the automated testing of a large panel of up to 260 commercially available drugs ex-vivo on the 3D organoids, focusing particularly on drugs targeting epigenetic modifications. This will allow the researchers to compare the differential responses of primary and recurrent gliomas to different concentrations of each individual drug and across multiple patients, thereby enabling patient classification based on their reactions to the various compounds. The most effective drug candidates for a given tumour organoid will then be validated in-vivo on the corresponding patient-specific PDOX models of primary and recurrent gliomas. This will enable the validation of the efficacy of the drug but also a preliminary assessment of whether the drug is able to enter the brain and how it is metabolised by the organism.From a clinical standpoint, the PFP procedure within GLASS-LUX will make it possible to identify which treatment or combination of treatments could be the most effective for a given patient according to both the specific genetic characteristics and, importantly, the recurrence state of the tumour, providing oncologists with concrete and personalised therapeutic advice for their patients.
GLASS-LUX will ultimately lay the foundations for a personalised clinical trial for patients with these incurable recurring gliomas. Moreover, our focus on epigenetic modifications will also allow us to identify biomarkers to predict the response of specific subgroups of patients to drugs targeting these epigenetic processes”, explains Prof Niclou, principal investigator of GLASS-LUX.
The project is also a perfect instance of translational ‘bed to bench to bed’ study, for PFP can be applied to other tumour types, making this approach a generalised concept for precision oncology. Indeed, together with other clinical partners, the LIH team has already started a pilot phase I clinical trial to translate this approach into the clinical setting for glioblastoma and colorectal cancer. In the future, depending on the clinical validation phase, PFP could even become a new theranostic tool (i.e. a combination of therapy and diagnostics) that can be commercialised in the form of a service offered in collaboration with clinical partners in Luxembourg and the Greater Region, thus improving the early diagnosis and treatment of recurrent gliomas.
GLASS-LUX benefits from the support of the international GLASS consortium and of its long-term clinical partners − namely the Neurosurgery Department of the Centre Hospitalier de Luxembourg, the National Centre of Pathology at LNS and the National Centre of Genetics at LNS – which will ensure the successful collection of patient glioma tissues and their detailed molecular and genetic analysis. The LIH team will consist of Dr Anna Golebiewska, Anais Oudin, Dr Yong-Jun Kwon, and Dr Petr Nazarov.

1. DNA modifications affecting gene activity but without altering the DNA sequence.